Translational and Clinical Research
Mass cytometry and Imaging Mass Cytometry use is expanding in clinical research trials
CyTOF® technology has been adopted for use in dozens of clinical research trials around the world to investigate multiple areas of human disease to understand and improve prevention and therapeutics.
Here’s why:
- 50-plus unique markers per panel maximize information from every precious patient sample.
- Cells stained with metal-tagged antibodies can be frozen, stored and shipped reliably.
- Panels are easier to adjust to accommodate new findings than those that are fluorescence-based.
- Multiple publications prove that CyTOF assays and instruments provide reliable and reproducible results.
The use of mass cytometry and Imaging Mass Cytometry™ in clinical trials continues to steadily grow and be standardized across trials related to cancer immunotherapies.
Download the CyTOF clinical trials flyer to learn details about the network involved in standardization of CyTOF in clinical trials and more.
Download now
Growing adoption of CyTOF technology in National Clinical Trials
207 Trials as of June 2022
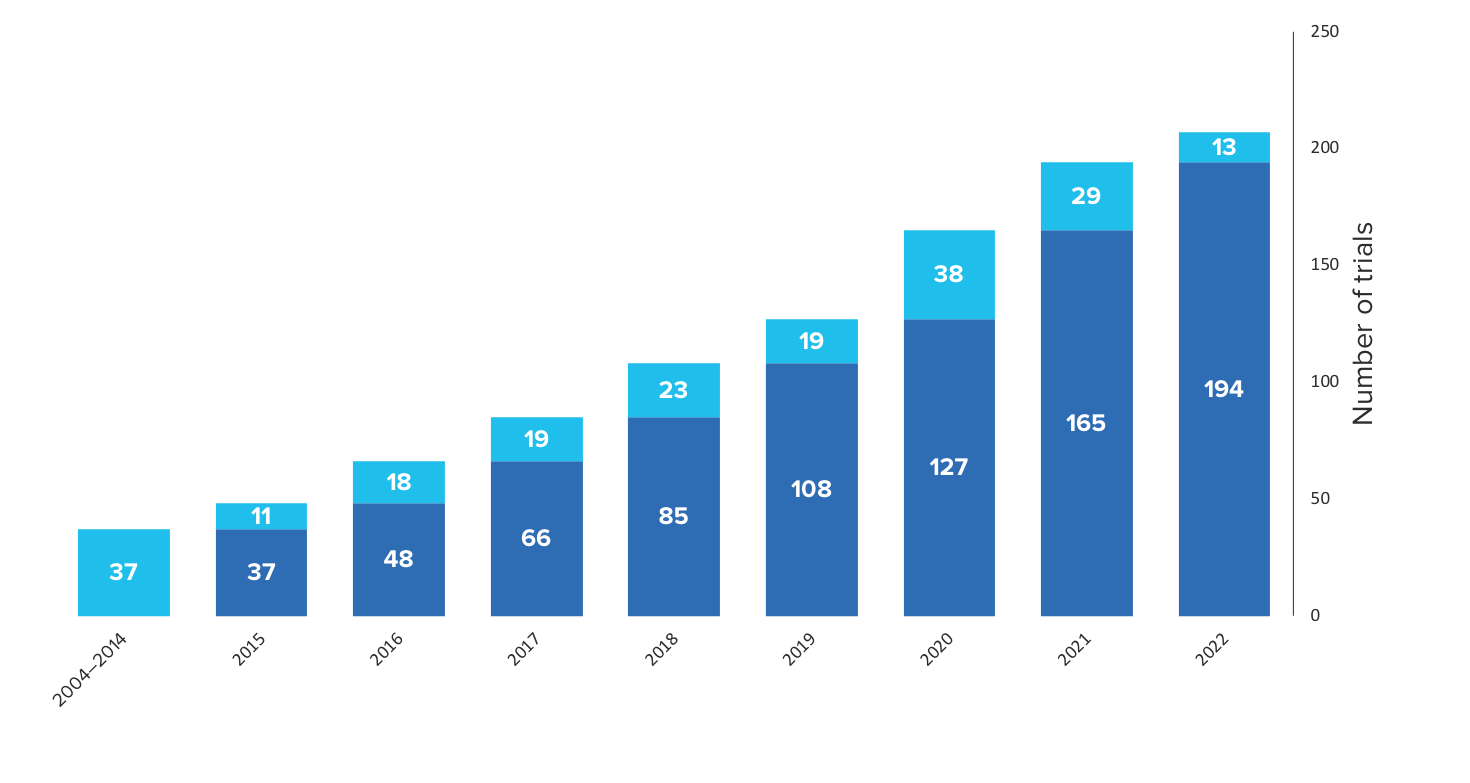
Sources: clinicaltrials.gov and various publications
National Clinical Trials citing CyTOF technology by research area
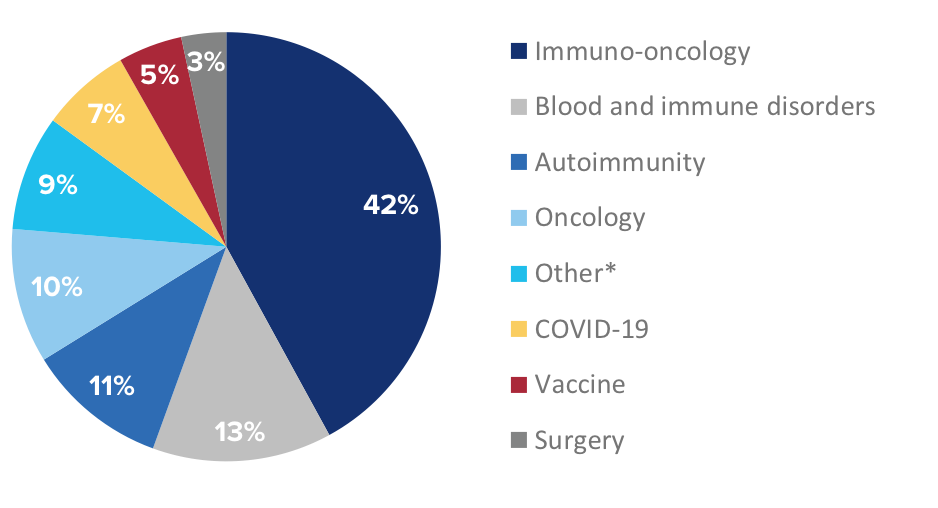
*Includes allergy, cardiac disease, pulmonary disease and stem cell
CyTOF® for drug development
Expand the questions you can ask and get the most from those small samples … Hear directly from CyTOF users about why they use mass cytometry for their immune profiling studies, from high-parameter needs in vaccine development to quantitative receptor analysis for drug development and immunotherapy testing.

WORKFLOW
1
Sample
Sample types:
- Whole blood
- PBMC
- Bone marrow
- Tissue digests
- Tumor digests
2

Stain, store, barcode
- Stain samples at multiple sites or on different days.
- Store, freeze and ship samples to a centralized location.
- Barcode samples to combine in a single tube.
3

Analyze
Analyze samples on CyTOF XT™ or Helios™ to measure 50 or more proteins in a single cell.
Webinars
Cutting Edge Cytometry and Clinical Trials: A Case Study
Method validation studies using the Maxpar® Direct™ Immune Profiling Assay™

Sasidhar Vemula, PhD
Lead Scientist
Translational Biomarker Solutions
Labcorp Drug Development
Vanderbilt University
CyTOF in the Cancer Immune Monitoring and Analysis Centers (CIMAC): standardization and harmonization

Holden Maecker, PhD
Professor of Microbiology and Immunology
Director of the Human Immune Monitoring Center, Stanford University
A complete immune monitoring solution with CyTOF: Ideal for pandemics and beyond
Leveraging CyTOF to track cancer vaccine induced T cell responses for up to 20 years

Erika J. Crosby, PhD
Center for Applied Therapeutics RCR/R&R Curriculum Manager, Duke University Medical Center
Using mass cytometry to identify clinically relevant biological signatures in human health and disease

Brice Gaudilliere, MD, PhD
Associate Professor, Departments of Anesthesiology, Perioperative & Pain Medicine and (by courtesy) of Pediatrics, Stanford University School of Medicine
Customer Stories
Learn more about how our customers are leveraging Standard BioTools™ technology
VIEW ALL
Wendy Fantl, PhD

On the right path to better ovarian cancer diagnosis and treatment
Lori Turner, PhD

A quick pivot to COVID-19 research with CyTOF
Hema Kothari, PhD

A lesson in how CyTOF technology can empower flexibility in research
Marcelo Sztein, MD

Research focuses on infection, vaccination in humans
RELATED BLOG POSTS
-
Trillions of cells, hundreds of cell types, one collaborative effort
Atlasing how so many cells work together to make us human

No CyTOF? No problem.
We can help you convert your fluorescence panel and find a service provider.
Unless explicitly and expressly stated otherwise, all products are provided for Research Use Only, not for use in diagnostic procedures. Find more information here.


